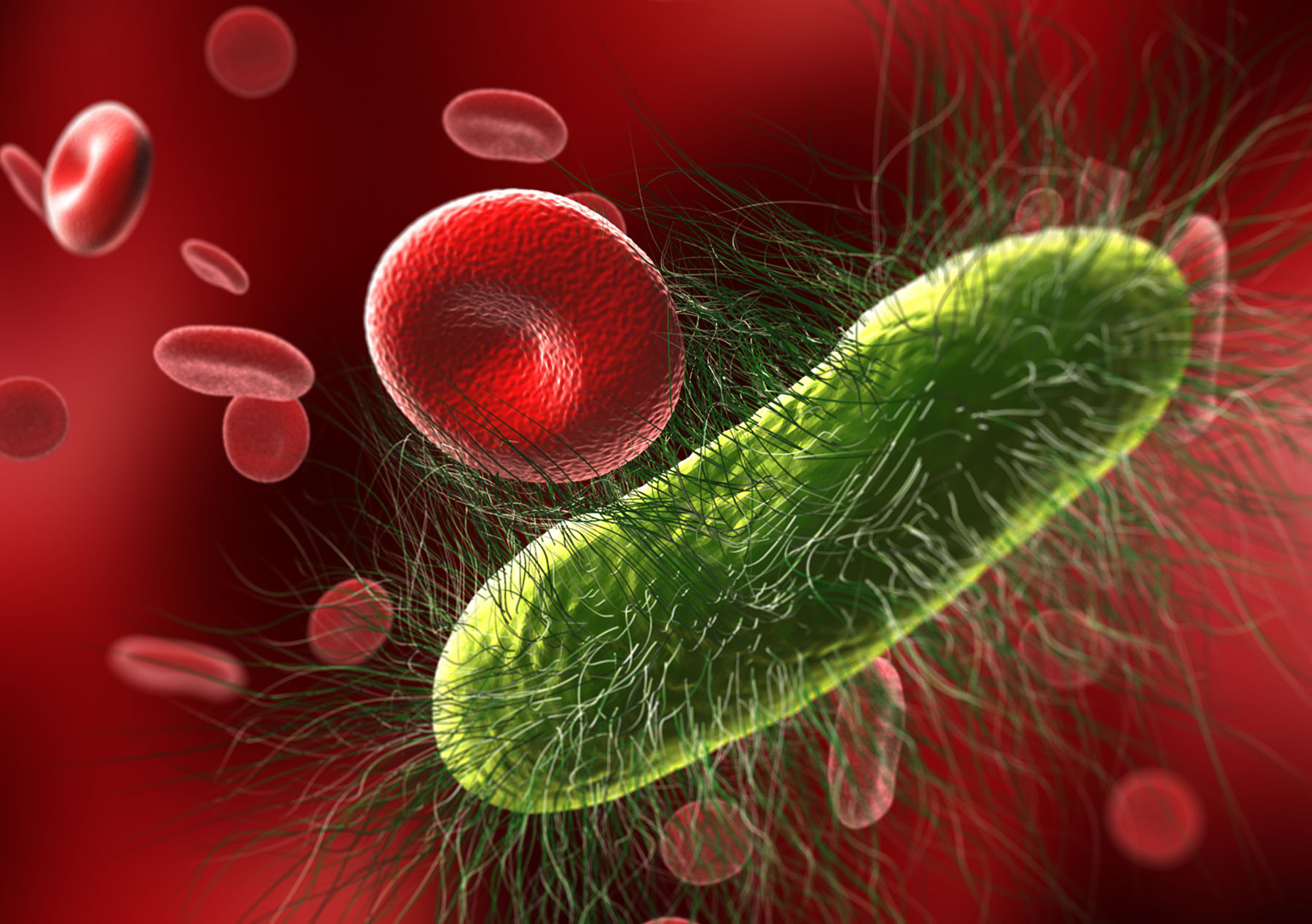
Biological Threat Agents
MRIGlobal’s services span all stages of the diagnostic product development process, beginning with research and development.
Our research, development, testing, and evaluation supporting biological threat agent disease diagnostics includes molecular and immunological assay method and platform development. Our work leads clients through clinical validation, including FDA 510(k), Pre-Market Notification (PMN), and CE Mark Submissions.
Capabilities
We develop assays and protocols related to biological threat disease diagnostics:
Molecular & Immunological
Next Generation Sequencing
Advanced Bioinformatics
Prototype development of sample collection, testing, preservation, and transport solutions for austere, nonconventional, and traditional laboratories
Our Staff
Theresa Seitz prepares a sample for testing on a point-of-care diagnostic device.
MRIGlobal scientists conduct research, development, testing, and evaluation to support biological threat disease diagnostic technology innovation. The goal of this support is to ultimately result in an FDA-cleared product.

Work with Us
Learn more about how MRIGlobal can test and evaluate your chemical and biological approaches by contacting us.
Contact us to learn more about how we can help with your next endeavor.
